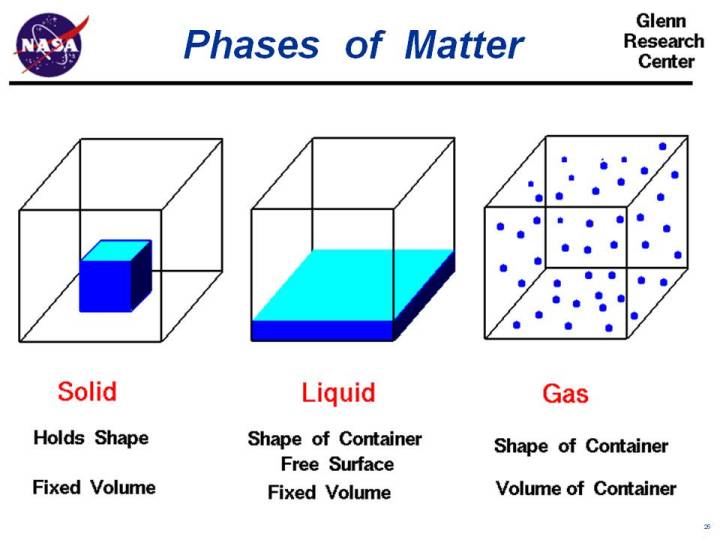
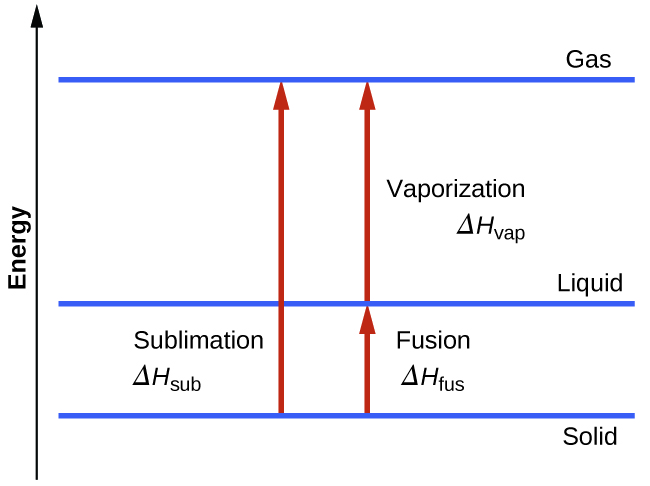
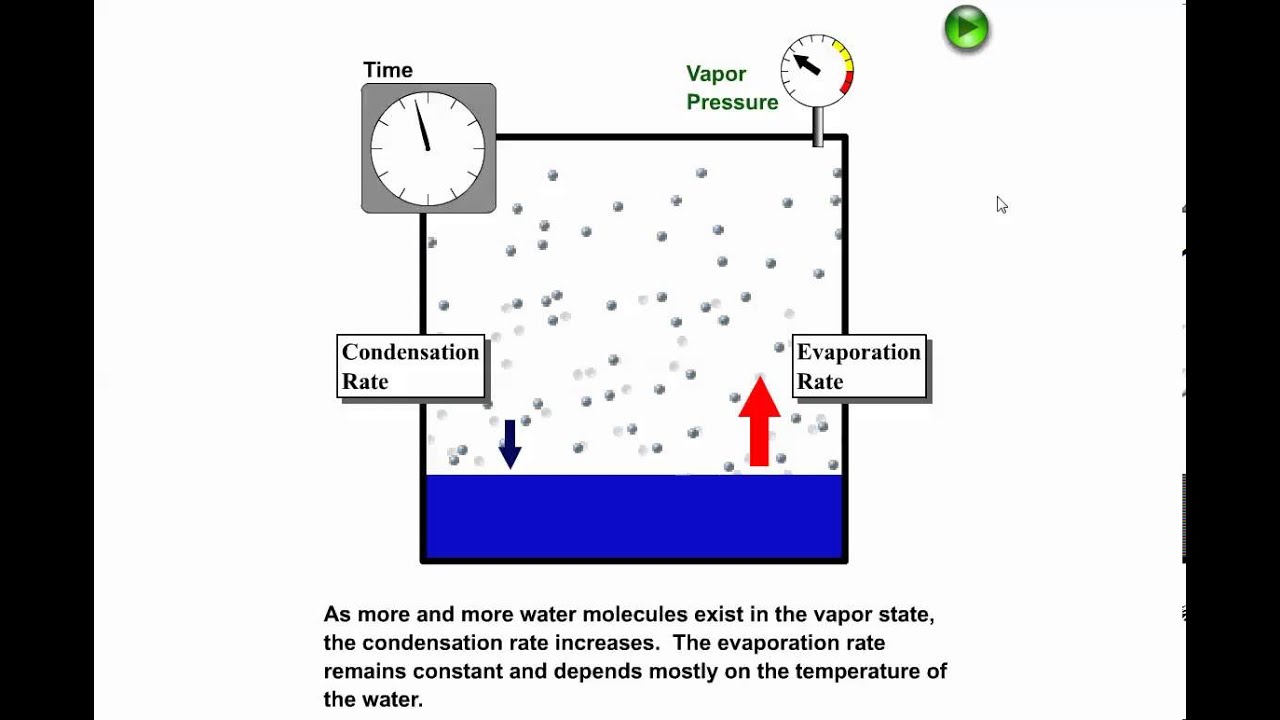
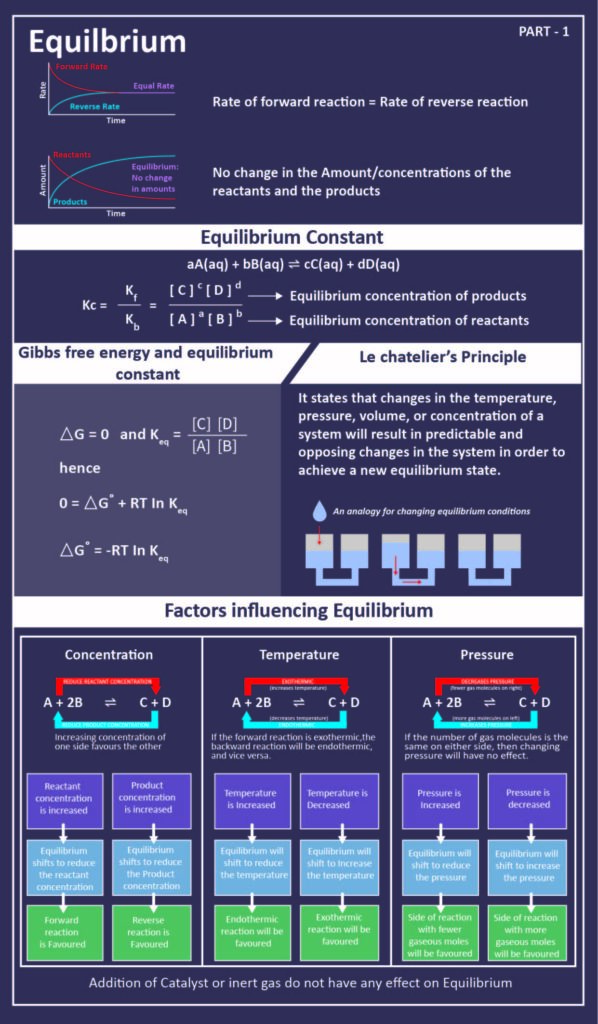
The term sublimation is the passage or the transformation or conversion that substances undergo when passing from one state to another for example from a solid substance to gas.
Transformation of solid iodine to the gaseous state at room temperature.
Iodine is a solid at room temperature because because at 114c it turns from a solid to a liquid.
Freezing is an example of an.
Sublimation occurs at a very low super cold temperature and low pressure.
The energy needed to change an amount of a substance from a solid to a liquid is the.
Is an endothermic process.
We can define sublimation as the transition of a substance from the solid phase to the gaseous phase without changing into the liquid phase.
Results in the reaction i2.
The process of transition of a substance directly from its solid phase to gaseous phase without going through the liquid state is called sublimation.
As a pure element iodine is a lustrous purple black nonmetal that is solid under standard conditions.
An endothermic reaction corresponds to one in which.
Sublimation is the process of direct transformation from the solid state to the gaseous state without passing through a liquid phase.
The temperature at which a solid becomes a liquid is called the.
This is a.
Some examples of sublimation chemistry are the processes that experience water carbon dioxide iodine arsenic or sulfur.
The density does change a lot when going from solid to gas.
Iodine is a chemical element with the symbol i and atomic number 53.
Iodine iodine physical and chemical properties.
The direct transformation of solid iodine to the gaseous state at room temperature.
It is an endothermic phase transition that occurs at temperatures and pressures below the triple point of a substance temperature and.
Is an endothermic process.
Does not affect the density of the iodine.
Results in a chemical change.
Is an endothermic process.
The molecular lattice contains discrete diatomic molecules which are also present in the molten and the gaseous states.
Iodine crystals dry ice naphthalene moth balls and arsenic tend to sublimate.
The temperature of a gas or vapor in its critical state.
Above 700 c 1 300 f dissociation into iodine atoms becomes appreciable.
Iodine is a nonmetallic nearly black solid at room temperature and has a glittering crystalline appearance.
The temperature of the sample remains constant while the ice melts.
It sublimes changes from a solid to a gaseous state while bypassing a liquid form easily.
Is a chemical property of iodine.