The usp purified water and the usp wfi on the other hand are components or ingredient materials as they are termed by the usp intended to be used in the production of drug products.
Usp purified water conductivity.
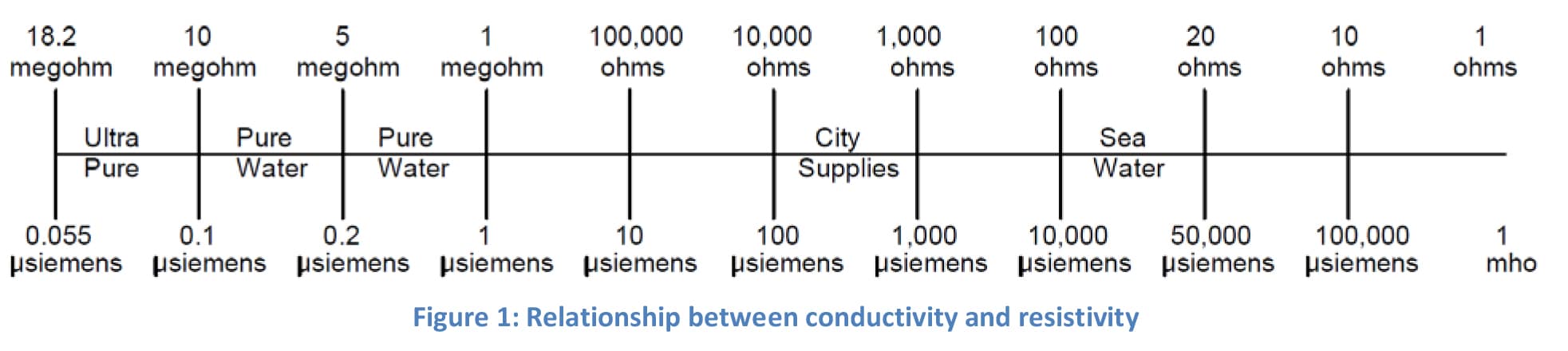
One is for the intrinsic conductivity of water and the other is for the other ionic species in water.
Note that you cannot fail.
The conductivity of the ubiquitous chloride ion at the theoretical endpoint concentration of 0 47 ppm when it was a required attribute test in usp xxii and earlier revisions and the ammonium ion at the limit of 0 3 ppm represents a major portion of the allowed water impurity level.
We can not provide photocopies of copyrighted material.
The heavy metals test on usp waters was deleted in 1996.
E g as described in iso 7888 water quality determination of electrical conductivity.
Yes this is correct.
Regardless of the font and letter case used in its spelling water complying with the purified water monograph is intended.
The usp is also available at pharmacy colleges.
1752 1754 and the general chapters 643 toc 645 water conductivity p.
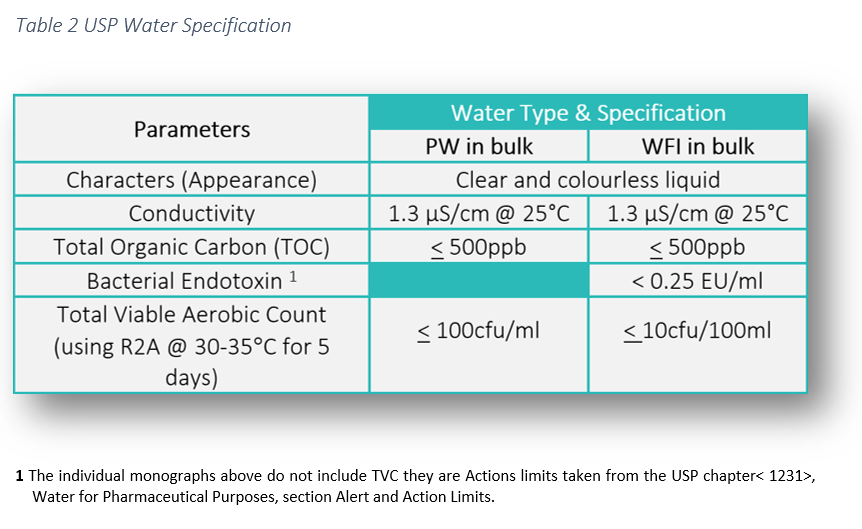
Water conductivity is used as a measure of purity for bulk usp purified water pw and water for injection wfi in the pharmaceutical industry.
Usp24 contains complete versions of all pharmaceutical water monographs p.
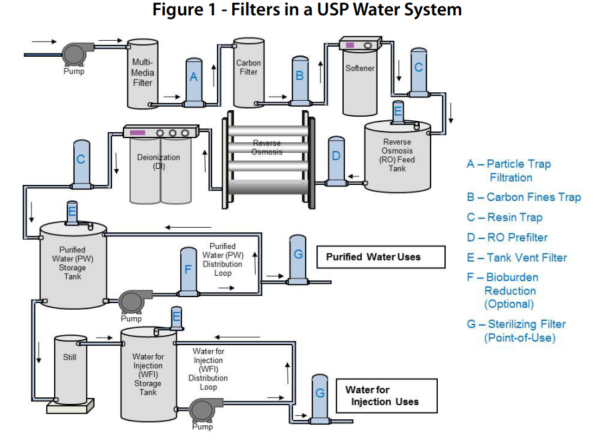
We have established a process purified water charcoal treatment softening uv sanitization and 0 2um filtration while the water quality is better than drinking water but not good enough to fulfill usp ep water specification only conductivity higher than the criteria 2 3us cm others are all within spec.
Purified water packaged in bulk for commercial use elsewhere meets the requirements of all of the tests under sterile purified water except labeling and sterility 71.
Including total vial count and.
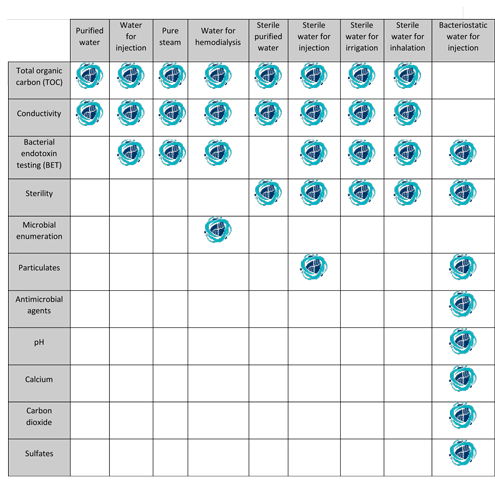
Purified water is also referenced throughout the usp nf.
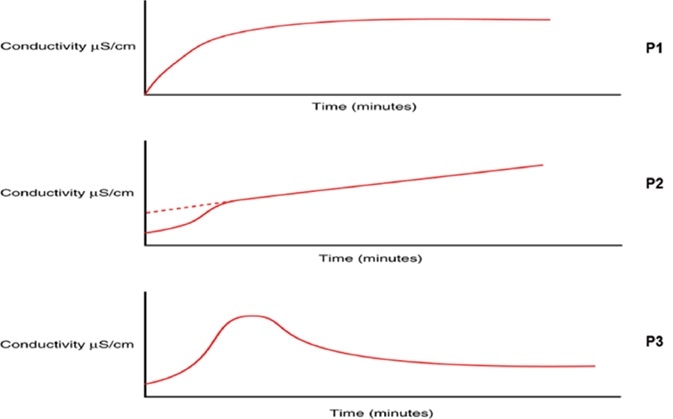
Note there is a ph measurement not a test as a part of stage 3 test for water conductivity 645 but this is still a conductivity limit test.
The tests for total organic carbon and conductivity apply to purified water produced on site for use as an ingredient of official preparations and in tests and assays.
The ph test was deleted in 1998.
For water packaged in bulk but manufactured elsewhere or for sterile purified water sterile water for injection sterile.
You may purchase usp24 by calling customer service at 800 877 6733.
The procedure described in the section bulk water is designed for measuring the conductivity of waters such as purified water water for injection water for hemodialysis and the condensate of pure steam produced in bulk.
In cases of very low conductivity 10 µs cm such as purified pharmaceutical waters two compensations need to be made.
Unless otherwise specified purified water is also to be used for all tests and assays for which water is indicated see general notices and requirements.
Conductivity testing is more reliable and.
There has never been a test for nitrates for usp waters.